It's a New Day in Public Health.
The Florida Department of Health works to protect, promote, and improve the health of all people in Florida through integrated state, county, and community efforts.
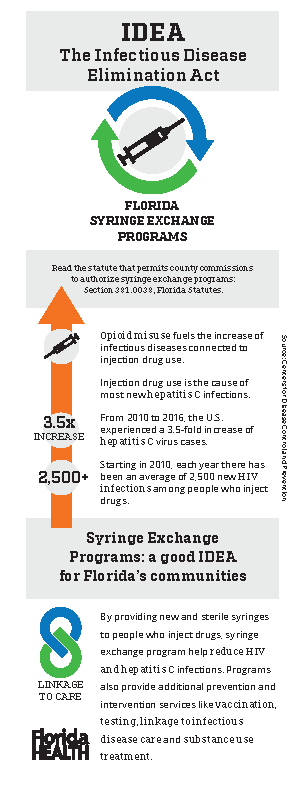
IDEA - Exchange
Florida Health

ESTABLISH
Before a syringe exchange program can be established, a county commission must:
1. Authorize the program under the provisions of a county ordinance.
2. Enter into a letter of agreement with the Florida Department of Health (FDOH), agreeing that the program will operate in accordance with 381.0038, Florida Statutes.
3. Partner with the county health department for consultation and recommendations for program operations.
4. Contract with one of the following to operate the program:
• A hospital licensed under Chapter 395, Florida Statutes.
• A health care clinic licensed under part X of Chapter 400, Florida Statutes.
• A Florida medical school accredited by the Liaison Committee on Medical Education or Commission on Osteopathic College Accreditation.
• A licensed addiction receiving facility as defined in s. 397.311, Florida Statutes.
• A 501(c)(3) HIV/AIDS service organization.
CREATE & MAINTAIN
To create a syringe exchange program, an operator must:
• Develop an oversight and accountability system that ensures compliance with Florida law and all contracts.
• Receive county commission approval of the oversight and accountability system before launching a program.
• Operate a one-to-one exchange—this means that participants can only receive one sterile syringe in exchange for a used one.
• Offer participants educational materials on HIV/AIDS, viral hepatitis and other blood-borne diseases.
• Provide on-site counseling or referrals for drug abuse prevention, education and treatment, and on-site screening or referrals for HIV and viral hepatitis testing and treatment.
• Make available kits, or referrals to programs that can provide kits, that contain emergency opioid antagonists.
REPORT
Syringe exchange programs must provide an annual report to county commissioners and FDOH by Aug. 1. The report must include:
• The number of participants served.
• The number of used needles and syringes received.
• The number of clean, unused needles and syringes distributed.
• Demographic profiles of the participants served.
• The number of participants entering drug counseling or treatment.
• The number of participants receiving testing for HIV, viral hepatitis and other blood-borne diseases.
• Other data required under FDOH rule. IDEA allows FDOH to develop rules for collecting data.




Connect with DOH