The Florida Department of Health declares they are ready to receive electronic case reports from eligible health care organizations.
Participate in eCR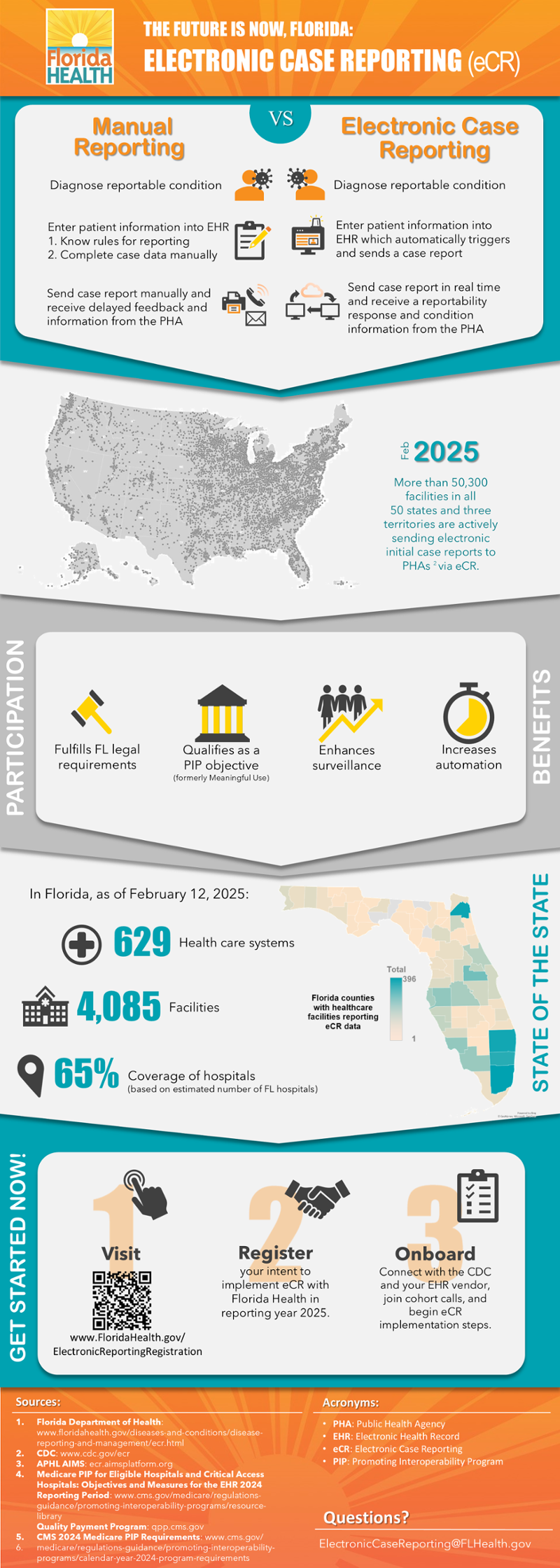
Effective January 1, 2022, eCR is required by the Centers for Medicare and Medicaid Services' Promoting Interoperability Program (PIP) for eligible hospitals and critical access hospitals (CAHs) and the Merit-Based Incentive Payment System (MIPS) Promoting Interoperability Performance Category for eligible clinicians.
PLEASE NOTE: The upcoming deadline for eligible hospitals, critical access hospitals (CAHs), and clinicians to begin the reporting/performance period to meet the CY2025 requirement for CMS PIP and QPP MIPS for eCR is July 5th, 2025. It must be a continuous, self-selected, 180-day period ending by December 31, 2025.
Please read on to learn about eCR, its benefits, where to register your intent, and additional resources.
About eCR
Electronic case reporting (eCR) is the automated real-time exchange of case report information between electronic health records (EHRs) and public health agencies.
Traditionally, health care providers need to know the rules for reporting and manually report about cases, or people with conditions of public health concern, to their public health agencies via fax, phone call, or email.
The Florida Department of Health uses the Health Level 7 (HL7) electronic initial case report (eICR) and Reportability Response (RR) standards for eCR. It is these standards that we will use to eventually eliminate manual reporting requirements. Please ensure that your Electronic Health Record (EHR) product is capable of supporting the HL7 implementation guides shown below:
- HL7 Clinical Document Architecture (CDA) implementation guides hl7.org/implement/standards/product_brief.cfm?product_id=436
- eICR R1.1 (HL7 CDA® R2 Implementation Guide: Public Health Case Report Release 2: the Electronic Initial Case Report (eICR) Release 1, STU Release 1.1 - US Realm)
- eICR R3.1.1 (HL7 CDA® R2 Implementation Guide: Public Health Case Report - the Electronic Initial Case Report (eICR) Release 2, STU Release 3.1.1 - US Realm)
- RR R1.1 (HL7 CDA® R2 Implementation Guide: Reportability Response, Release 1, STU Release 1.1 - US Realm)
- HL7 Fast Healthcare Interoperability Resources (FHIR) Implementation Guide hl7.org/fhir/us/ecr/STU2.1
- FHIR eCR R2.1.2 (HL7 FHIR® Implementation Guide: Electronic Case Reporting (eCR), Release 2.1.2 - US Realm)
Benefits of eCR
- Fulfills legal requirements: eCR allows providers to fulfill their legal obligations to report conditions of public health concern with less administrative burden.
- Qualifies as a PIP objective: eCR qualifies as an objective for eligible facilities participating in the Promoting Interoperability Program (PIP), formerly called Meaningful Use.
- Enhances surveillance: eCR enhances the efficiency and effectiveness of disease detection, surveillance, investigation, and response.
- Increases automation: eCR increases automation, which allows stakeholders to spend more time providing care and preventing and controlling disease among the residents of Florida, especially those who are most vulnerable.



Connect with DOH